| Remove | Remove | Remove | |
 |
 |
 |
|
| Title | Raloxifene Impurity 9 | Bilastine Impurity 27 | Posaconazole Impurity 22 |
| Add to cart |
|
|
|
| Description |
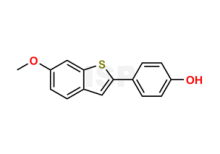
Raloxifene Impurity 9 is chemically 6-Methoxy-2-(4-hydroxyphenyl)benzo[b]thiophene; 4-(6-Methoxybenzo[b]thien-2-yl)phenol;. The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ISP Standards products are for analytical purpose only and not for human use. |
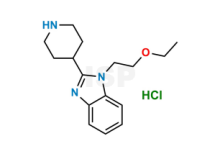
Bilastine Impurity 27 is chemically 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride. The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ISP Standards products are for analytical purpose only and not for human use. |
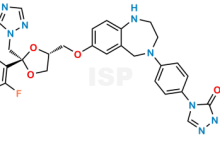
Posaconazole Impurity 22 is chemically D-threo-Pentitol, 2,5-anhydro-1,3,4-trideoxy-2-C-(2,4-difluorophenyl)-4-[[[4-[4-[1-[(1S,2S)-1-ethyl-2-hydroxypropyl]-1,5-dihydro-5-oxo-4H-1,2,4-triazol-4-yl]phenyl]-2,3,4,5-tetrahydro-1H-1,4-benzodiazepin-7-yl]oxy]methyl]-1-(1H-1,2,4-triazol-1-yl)-;. The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ISP Standards products are for analytical purpose only and not for human use. |
| Availability | In stock | In stock | In stock |
| CAS No | 175460-94-3 | 1841081-72-8 | 1388148-30-8 |
| Inv. Status | Out of Stock | In Stock | Custom Synthesis |
| Mol.F. | C15H12O2S | C16H23N3O : HCl | C36H40F2N8O4 |
| ISP CAT No | ISP-R007017 | ISP-B034040 | ISP-P022042 |
| Mol.Wt. | 256.3 | 273.4 : 36.5 | 686.8 |