| Remove | Remove | Remove | Remove | |
 |
 |
 |
 |
|
| Title | Vitamin K1 Chromenol Impurity | Mebeverine EP Impurity O | Didesmethyl Hydroxy Cariprazine | Diltiazem N,N-DiDesmethyl TFA Salt |
| Add to cart |
|
|
|
|
| Description |
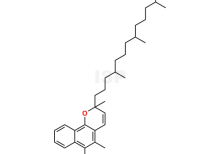
Vitamin K1 Chromenol Impurity is chemically 2,5-Dimethyl-2-(4,8,12-trimethyltridecyl)-2H-benzo[h]chromen-6-ol. The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ISP Standards products are for analytical purpose only and not for human use. |
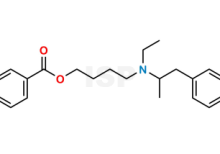
Mebeverine EP Impurity O is chemically 4-[Ethyl[(2RS)-1-(4-methoxy-3-methylphenyl)propan-2-yl]amino]butyl 3,4-dimethoxybenzoate (as per EP). The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ISP Standards products are for analytical purpose only and not for human use. |
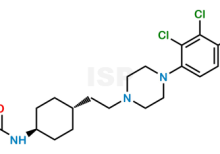
Didesmethyl Hydroxy Cariprazine is chemically 1-((1r,4r)-4-(2-(4-(2,3-Dichloro-4-hydroxyphenyl)piperazin-1-yl)ethyl)cyclohexyl)urea. The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ISP Standards products are for analytical purpose only and not for human use. |
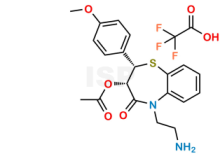
Diltiazem N,N-DiDesmethyl TFA Salt is chemically (2S,3S)-5-(2-Aminoethyl)-2-(4-methoxyphenyl)-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]thiazepin-3-yl acetate 2,2,2-trifluoroacetate. The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ISP Standards products are for analytical purpose only and not for human use. |
| Availability | In stock | In stock | In stock | In stock |
| CAS No | 34044-00-3 | 2512206-37-8 | 1084891-93-9 | |
| Inv. Status | In Stock | Out of Stock, Custom Synthesis | Out of Stock, Custom Synthesis | Out of Stock, Custom Synthesis |
| Mol.F. | C31H46O2 | C26H37NO5 | C19H28Cl2N4O2 | C20H22N2O4S : C2HF3O2 |
| ISP CAT No | ISP-P061018 | ISP-M054014 | ||
| Mol.Wt. | 450.7 | 443.6 | 415.4 | 386.5 : 114.0 |