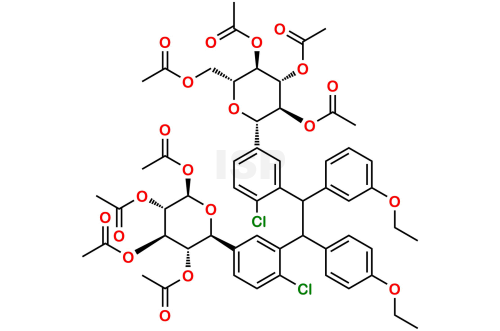
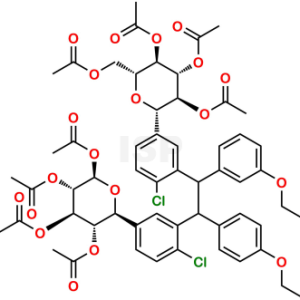
Dapagliflozin Dimer Impurity
| Mol.F. | |
|---|---|
| Mol.Wt. | |
| Inv. Status |
Disclaimer
The product information provided on this website by ISPStandards is based on the best available understanding at the time of publication. Customers are responsible for verifying the accuracy of this information before making a purchase. ISPStandards may update product details as new information or specifications become available, without prior notice.
- Product Overview
- Description
- Technical Data
Description
Chemical Name: ((1,2 Bis-(Ethoxy phenyl),1’,2;-bis-((2R,3R,4R,5S,6S)-2-(Acetoxymethyl)-6-[4-chlorophenyl]tetrahydro-2H-pyran-3,4,5-triyl triacet ate) ethane
Shipping Temperature:
Ambient
HSN Code:
38229010
Country of Origin: India
Smiles: ClC1=CC=C([C@@H]2O[C@H](OC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]2OC(C)=O)C=C1C(C3=CC=C(OCC)C=C3)C(C4=CC(OCC)=CC=C4)C5=CC([C@@H]6O[C@H](COC(C)=O)[C@@H](OC(C)=O)[C@H](OC(C)=O)[C@H]6OC(C)=O)=CC=C5Cl
Additional Information
Dapagliflozin Dimer Impurity is chemically ((1,2 Bis-(Ethoxy phenyl),1’,2;-bis-((2R,3R,4R,5S,6S)-2-(Acetoxymethyl)-6-[4-chlorophenyl]tetrahydro-2H-pyran-3,4,5-triyl triacet ate) ethane .
Dapagliflozin Dimer Impurity is supplied with detailed characterization data compliant with regulatory guideline. Dapagliflozin Dimer Impurity can be used for the analytical method development, method validation (AMV), Quality Controlled (QC) application for Abbreviated New Drug Application (ANDA) or during commercial production of Dapagliflozin.
The product can be used as reference standards and further traceability against pharmacopeial standards (USP or EP) can be provided based on feasibility. ISP Standards products are for analytical purpose only and not for human use.
NA